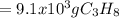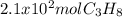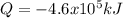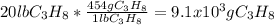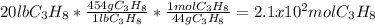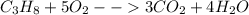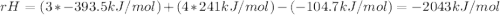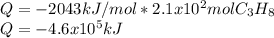How many grams of propane are in 20 pounds of propane? use the conversion 1 lb = 454 g. (express your answers for the next three questions in scientific notation. for example use 2.3e-5 to indicate a number such as 2.3 x 10-5.) 9.1e3 grams b) how many moles of propane are in 20 pounds of propane? 2.1e2 moles c)how much heat can be obtained by burning 20 pounds of propane? (remember to look at this from the viewpoint of the surroundings, since the question asks how much heat can be obtained.) 4.6e5 kj

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 23.06.2019 15:30, kfull6027
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3 +(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1

Chemistry, 23.06.2019 15:30, rociomartinez9
In most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of in most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of potential energy; kinetic energy kinetic energy; potential energy the energy of motion; stored energy chemical work; energy stored in chemical bonds
Answers: 2
Do you know the correct answer?
How many grams of propane are in 20 pounds of propane? use the conversion 1 lb = 454 g. (express yo...
Questions in other subjects:

Social Studies, 31.07.2019 02:40

History, 31.07.2019 02:40

History, 31.07.2019 02:40

Mathematics, 31.07.2019 02:40

History, 31.07.2019 02:40

Health, 31.07.2019 02:40

SAT, 31.07.2019 02:40


Health, 31.07.2019 02:40