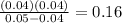Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
Do you know the correct answer?
Trichloroacetic acid (ccl3co2h) is a corrosive acid that is used to precipitate proteins. the ph of...
Questions in other subjects:

Mathematics, 13.09.2021 04:00

English, 13.09.2021 04:00


Mathematics, 13.09.2021 04:00

Chemistry, 13.09.2021 04:00



English, 13.09.2021 04:00

Mathematics, 13.09.2021 04:00


![\frac{[H^{+}][ClO_{4}^{-}]}{[HClO_{4}]}](/tpl/images/0356/4769/5addd.png)