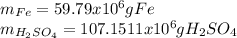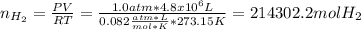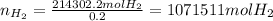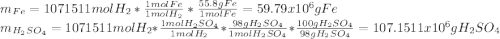Chemistry, 02.11.2019 04:31, giavanleer14
In 1897 the swedish explorer andreé tried to reach the north pole in a balloon. the balloon was filled with hydrogen gas. the hydrogen gas was prepared from iron splints and diluted sulfuric acid. the reaction is
fe(s)+ h2so_4(aq) > feso4(aq) + h2(g)
the volume of the balloon was 4800 m^3, and the loss of hydrogen gas during filling was estimated at 20.%. what mass of iron splints and 98% (by mass) h2so4 were needed to ensure the complete filling of the balloon? assume a temperature of 0^c, a pressure of 1.0 atm during filling, and 100% yield

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Do you know the correct answer?
In 1897 the swedish explorer andreé tried to reach the north pole in a balloon. the balloon was fill...
Questions in other subjects:

Mathematics, 26.05.2020 22:57


Mathematics, 26.05.2020 22:57

Spanish, 26.05.2020 22:57


Mathematics, 26.05.2020 22:57


History, 26.05.2020 22:57