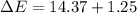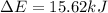Chemistry, 02.11.2019 03:31, Jsanders2276
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperature of 35.2 °c caused the liquid to vaporize (change to a gas). the vaporized gas expanded against an external pressure of 1.07 atm and a volume change of 11.49 l was observed. (recall: 1 l• atm = 101.3 j} what was the change in the internal energy of the system, (ae in kj)? (enter your answer with two decimals places and no units.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperatu...
Questions in other subjects:

Social Studies, 16.11.2019 09:31


Physics, 16.11.2019 09:31

History, 16.11.2019 09:31



Biology, 16.11.2019 09:31

Mathematics, 16.11.2019 09:31

History, 16.11.2019 09:31


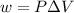
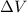 = change in volume = 11.49 L
= change in volume = 11.49 L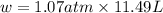
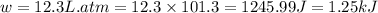 (as per conversion)
(as per conversion)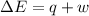
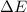 = internal energy of the system
= internal energy of the system