
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)2kcl(s) + 3o2(g) the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 749 mm hg. if the wet o2 gas formed occupies a volume of 5.76 l, the number of moles of kclo3 reacted was mol. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo...
Questions in other subjects:



Mathematics, 22.03.2020 18:37

Mathematics, 22.03.2020 18:37






History, 22.03.2020 18:42


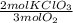 = 0,15 mol of KClO₃
= 0,15 mol of KClO₃



