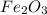Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
dur...

Chemistry, 24.01.2020 14:31, goverton101
Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
during this reaction, the oxidation number of fe changes from
(1) +2 to 0 as electrons are transferred
(2) +2 to 0 as protons are transferred
(3) +3 to 0 as electrons are transferred
(4) +3 to 0 as protons are transferred

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2


Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30


Computers and Technology, 17.02.2021 23:30


Mathematics, 17.02.2021 23:30

Mathematics, 17.02.2021 23:30