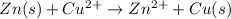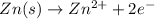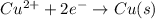Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation r...

Chemistry, 22.01.2020 03:31, alyssasnyderrr
Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation represents the oxidation half reaction?
(1) zn(s) + 2e– → zn2+(aq)
(2) zn(s) → zn2+(aq) + 2e–
(3) cu2+(aq) → cu(s) + 2e–
(4) cu2+(aq) + 2e– → cu(s)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, officialgraciela67
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1



Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
Do you know the correct answer?
Questions in other subjects:


English, 01.01.2021 09:00

Computers and Technology, 01.01.2021 09:00



Spanish, 01.01.2021 09:00



Mathematics, 01.01.2021 09:00