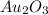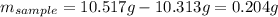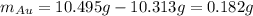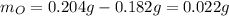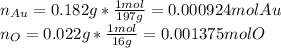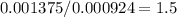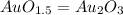Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean crucible that has a mass of 10.313 g. the crucible and sample have a mass of 10.517 g. the crucible is heated until the compound decomposes to the elements. the oxygen is lost to the air and the gold remains in the crucible. the mass of the crucible and gold is 10.495 g. what is the empirical formula of this compound?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 23.06.2019 04:40, dd123984
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
Do you know the correct answer?
Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean c...
Questions in other subjects:


History, 05.02.2021 19:00


Mathematics, 05.02.2021 19:00




Biology, 05.02.2021 19:00


Geography, 05.02.2021 19:00