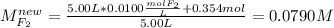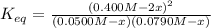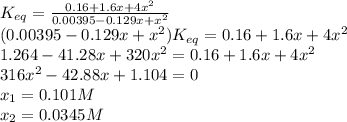Chemistry, 01.11.2019 02:31, krandall232
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in a 5.00 l rigid container are [h2] = 0.0500 m, [f2] = 0.0100 m, and [hf] = 0.400 m. if 0.345 mol of f2 is added to this equilibrium mixture, calculate the concentrations of all gases once equilibrium is reestablished in moles/liter. h2(g) + f2(g) < > 2 hf(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 04:50, mia36492
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table. state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
Do you know the correct answer?
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in...
Questions in other subjects:

History, 24.09.2019 20:30

Spanish, 24.09.2019 20:30


Geography, 24.09.2019 20:30





Physics, 24.09.2019 20:30

History, 24.09.2019 20:30


![[HF]_{eq}=0.469M, [H_2]_{eq}=0.0155M, [F_2]_{eq}=0.0445M](/tpl/images/0355/0697/87639.png)
![K_{eq}=\frac{[HF]^2}{[H_2][F_2]}=\frac{(0.400M)^2}{(0.0500M)(0.0100M)} =320](/tpl/images/0355/0697/ba1f2.png)