
Chemistry, 01.11.2019 02:31, luiscastaenda
Uman blood is kept at a typical ph of 7.40 mainly by the carbonic acid–carbonate ion buffer system. the corresponding chemical equation describing the buffer would be: h2co3(aq) h2o(l) ⇌ hco3-(aq) h3o (aq) the appropriate ka value for carbonic acid is 4.3×10-7, so its pka value is 6.37. calculate the [base]/[acid] ratio in human blood. (answer to 3 significant figures, no units)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
Do you know the correct answer?
Uman blood is kept at a typical ph of 7.40 mainly by the carbonic acid–carbonate ion buffer system....
Questions in other subjects:

Biology, 25.07.2019 19:00







Social Studies, 25.07.2019 19:00

Social Studies, 25.07.2019 19:00

History, 25.07.2019 19:00


![\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]}](/tpl/images/0355/0720/887f9.png) , is 10.7
, is 10.7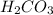 is an weak acid and
is an weak acid and 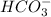 is it's conjugate base.
is it's conjugate base.![pH=pK_{a}(H_{2}CO_{3})+log(\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]})](/tpl/images/0355/0720/8cc41.png)
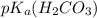 =6.37
=6.37![7.40=6.37+log(\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]})](/tpl/images/0355/0720/a095c.png)
![\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]}=10.7](/tpl/images/0355/0720/0e463.png)




