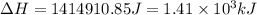Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam at 125 ∘c. the following constants may be useful: cm (ice)=36.57 j/(mol⋅∘c) cm (water)=75.40 j/(mol⋅∘c) cm (steam)=36.04 j/(mol⋅∘c) δhfus=+6.01 kj/mol δhvap=+40.67 kj/mol express your answer with the appropriate units. view available hint(s)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
Do you know the correct answer?
Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam...
Questions in other subjects:


English, 23.10.2019 18:30


History, 23.10.2019 18:30


English, 23.10.2019 18:30





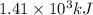
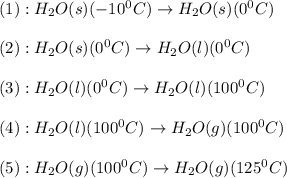
![\Delta H=[n\times c_{ice}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[n\times c_{water}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[n\times c_{steam}\times (T_{final}-T_{initial})]](/tpl/images/0355/0743/9dcae.png)
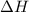 = enthalpy change = ?
= enthalpy change = ?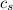 = specific heat of ice =
= specific heat of ice = 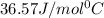
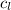 = specific heat of water =
= specific heat of water = 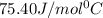
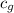 = specific heat of steam =
= specific heat of steam = 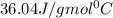
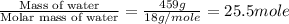
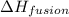 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole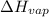 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25.5mole\times 36.57J/mol^0C\times (0-(-10))^0C]+25.5mole\times 6010J/mole+[25.5mole\times 75.40J/mol^0C\times (100-0)^0C]+25.5mole\times 40670J/mole+[25.5mole\times 36.04J/gmol^0C\times (125-100)^0c]](/tpl/images/0355/0743/cfaa1.png)