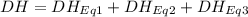At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj eq. 2: 2 o3(g) → 3 o2(g) δh = –284.6 kj eq. 3: 2 o2(g) → 4 o(g) δh = –990.0 kj use the above data to calculate the heat absorbed (kj) when 2.5 moles of no2(g) is formed at room temperature according to the chemical reaction. do not include units. no(g) + o(g) → no2(g)

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
Do you know the correct answer?
At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2...
Questions in other subjects:

Mathematics, 09.06.2021 15:40

History, 09.06.2021 15:40

Mathematics, 09.06.2021 15:40


English, 09.06.2021 15:40

History, 09.06.2021 15:40


Computers and Technology, 09.06.2021 15:40

Mathematics, 09.06.2021 15:40