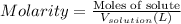A3.0 m hcl(aq) solution contains a total of
(1) 3.0 grams of hcl per liter of water
(2)...

Chemistry, 08.12.2019 14:31, carlosluiscontrerasp
A3.0 m hcl(aq) solution contains a total of
(1) 3.0 grams of hcl per liter of water
(2) 3.0 grams of hcl per mole of solution
(3) 3.0 moles of hcl per liter of solution
(4) 3.0 moles of hcl per mole of water

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Geography, 10.07.2019 11:00


Mathematics, 10.07.2019 11:00



History, 10.07.2019 11:00


Mathematics, 10.07.2019 11:00