
Chemistry, 31.10.2019 01:31, CaraRose1887
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both measured at 315 k and 50.0 mmhg), what is the limiting reactant and the theoretical yield of so3? if 187.2 ml of so3 is collected (measured at 315 k and 50.0 mmhg), what is the percent yield for the reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
Do you know the correct answer?
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both...
Questions in other subjects:




Mathematics, 07.04.2021 20:40

Mathematics, 07.04.2021 20:40

Engineering, 07.04.2021 20:40




Mathematics, 07.04.2021 20:40


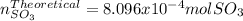
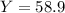 %
%
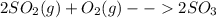
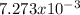 moles of
moles of 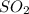 as follows:
as follows: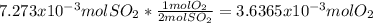
 moles are available in comparison with the
moles are available in comparison with the 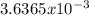 moles that completely would react with
moles that completely would react with 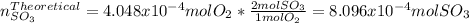
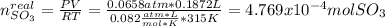
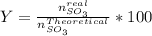 %
%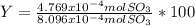 %
%



