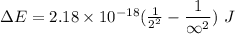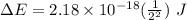Chemistry, 30.10.2019 05:31, htiffany0225
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially in the n =2 energy level. the energy needed to ionize a ground-state hydrogen atom is 2.18 x 10–18 j.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
Do you know the correct answer?
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially i...
Questions in other subjects:

History, 21.09.2019 09:10





Business, 21.09.2019 09:10


Health, 21.09.2019 09:10

Mathematics, 21.09.2019 09:10

Social Studies, 21.09.2019 09:10


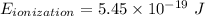
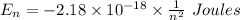
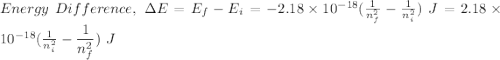
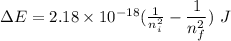
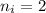 and
and 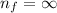 (As the hydrogen has to ionize)
(As the hydrogen has to ionize)