Chemistry, 30.10.2019 03:31, richtercatrina16
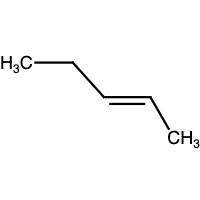
Compound x and compound y are constitutional isomers with the molecular formula c5h10. compound x possesses a carbon-carbon double bond in the trans configuration, while compound y possesses a carbon-carbon double bond that is not stereoisomeric: get answering molecular drawing questions get answering molecular drawing questions. draw the structure of compound x.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
Compound x and compound y are constitutional isomers with the molecular formula c5h10. compound x po...
Questions in other subjects:






English, 30.10.2020 16:50



Mathematics, 30.10.2020 16:50

Mathematics, 30.10.2020 16:50