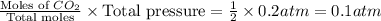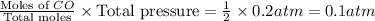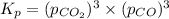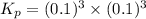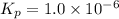When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3 co2(g) + 3 co(g) starting with just the oxalate in a 10.0 l flask, at equilibrium the total pressure observed is 0.200 atm. what is the value of kp for the equilibrium? (dalton’s law of partial pressure! )

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1


Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
Do you know the correct answer?
When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3...
Questions in other subjects:



English, 17.04.2021 01:00





History, 17.04.2021 01:00

Mathematics, 17.04.2021 01:00

Advanced Placement (AP), 17.04.2021 01:00


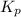 for the equilibrium is
for the equilibrium is 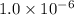
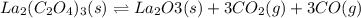
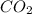 and
and 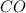 .
.