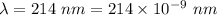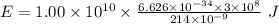Chemistry, 30.10.2019 03:31, lilyella1004
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 1.00×1010 atoms of zinc emitting light in the instrument flame at any given instant, what energy (in joules) must the flame continuously supply to achieve this level of emission? express your answer numerically in joules.
part b during an emission, electrons move from a higher energy orbital to a lower energy orbital. which of the following are valid transitions that produce lines in the emission spectrum of zn?
check all that apply.
1-[ar]4s13d106s1→[ar]4s23d10
2-[ar]4s23d10→[ar]4s23d104p2
3-[ar]4s23d10→[ar]3d10
4-[ar]4s23d10→[ar]4s13d11
5-[ar]3d10→[ar]4s23d10
6-[ar]4s23d10→[ar]4s13d106s1

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1


Chemistry, 23.06.2019 11:30, elizebeth4501
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
Do you know the correct answer?
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if...
Questions in other subjects:


Mathematics, 01.09.2019 02:20



Mathematics, 01.09.2019 02:20


Physics, 01.09.2019 02:20

History, 01.09.2019 02:20

Mathematics, 01.09.2019 02:20

English, 01.09.2019 02:20


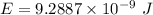
![[Ar]4s^13d^{10}6s^1\rightarrow [Ar]4s^23d^{10}](/tpl/images/0352/1669/c1431.png)
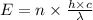
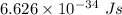
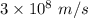
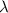 is the wavelength
is the wavelength