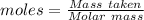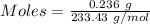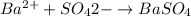Chemistry, 30.10.2019 03:31, putaprincess16
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the solution, a white precipitate forms. the precipitate is filtered and dried and then found to have a mass of 236 mg. what mass of barium was in the original solution? (assume that all of the barium was precipitated out of solution by the reaction.)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
Do you know the correct answer?
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the sol...
Questions in other subjects:



Chemistry, 14.08.2020 02:01



Mathematics, 14.08.2020 02:01

Mathematics, 14.08.2020 02:01



Mathematics, 14.08.2020 02:01


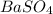 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg