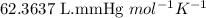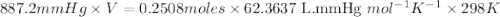Chemistry, 30.10.2019 01:31, scottmichetti
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction include aqueous calcium chloride, liquid water, and gaseous carbon dioxide. calculate the volume of co₂ gas collected over water at 25.0 °c when 25.1 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm hg. the vapor pressure of water at 25.0 °c is 23.8 mm hg.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1
Do you know the correct answer?
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction...
Questions in other subjects:




English, 10.03.2020 23:26

Biology, 10.03.2020 23:26






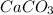 = 100.0869 g/mol
= 100.0869 g/mol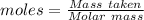
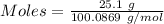
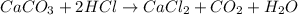
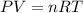
![25^oC=[25+273]K=298K](/tpl/images/0351/9682/df1f6.png)