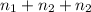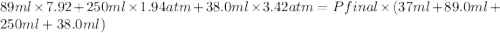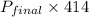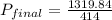Three bulbs are connected by tubing, and the tubing is evacuated. the volume of the tubing is 37.0 ml. the first bulb has a volume of 89.0 ml and contains 7.92 atm of argon, the second bulb has a volume of 250 ml and contains 1.94 atm of neon, and the third bulb has a volume of 38.0 ml and contains 3.42 atm of hydrogen. if the stopcocks (valves) that isolate all three bulbs are opened, what is the final pressure of the whole system in atm?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 05:00, xxaurorabluexx
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
Do you know the correct answer?
Three bulbs are connected by tubing, and the tubing is evacuated. the volume of the tubing is 37.0 m...
Questions in other subjects:











 ............... (1)
............... (1)