
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) the base dissociation constant for ammonia (nh3) is kb = 1.8 × 10–5. ammonia (nh3) also has a chloride salt, ammonium chloride (nh4cl), which is soluble in water. if 0.070 m of ammonia (nh3) and 0.035 m of its salt ammonium chloride (nh4cl) are mixed in a solution, what is the ph of this solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
Do you know the correct answer?
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) th...
Questions in other subjects:


Mathematics, 22.04.2021 19:10



History, 22.04.2021 19:10

Mathematics, 22.04.2021 19:10

English, 22.04.2021 19:10

Mathematics, 22.04.2021 19:10

Business, 22.04.2021 19:10

Mathematics, 22.04.2021 19:10


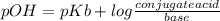
![pOH = pKb + log\frac{[NH_{4}^{+} ]}{[NH_{3}]} =4.7+log\frac{0.035M}{0.070M} =4.4](/tpl/images/0351/7427/0637f.png)




