
You have a mixture of 3-fluorophenol and ethyl-4-aminobenzoate that you wish to separate by extraction. the mixture is dissolved in methylene chloride. why can you not use nahco3 to separate one component from the other? (explain why nahco3 cannot either component into the aqueous layer).

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
Do you know the correct answer?
You have a mixture of 3-fluorophenol and ethyl-4-aminobenzoate that you wish to separate by extracti...
Questions in other subjects:











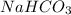 contains majorly
contains majorly 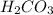 in solution.Ethyl-4-amino benzoate does not suffer side reaction e.g. ester hydrolysis with
in solution.Ethyl-4-amino benzoate does not suffer side reaction e.g. ester hydrolysis with 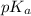 value around 8.4 and
value around 8.4 and 




