
Chemistry, 29.10.2019 21:31, Adolfosbaby
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar mass = 326.13 g/mol) was dissolved in water. the solution was titrated with kio3 , producing the precipitates la(io3)3 and ce(io3)3 . for the complete titration of both la3+ and ce3+ , 42.81 ml of 0.1250 m kio3 was required. calculate the mass fraction of la and ce in the sample.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1


Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
Do you know the correct answer?
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar...
Questions in other subjects:

Mathematics, 09.05.2021 03:40

Advanced Placement (AP), 09.05.2021 03:40


Mathematics, 09.05.2021 03:40


Mathematics, 09.05.2021 03:40


Physics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40

Mathematics, 09.05.2021 03:40


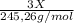 +
+ 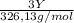 (1)
(1) ×
×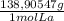 = 0,1022g of La
= 0,1022g of La  ×
×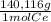 = 0,1468g of Ce
= 0,1468g of Ce 



