
Chemistry, 29.10.2019 19:31, yasameenakbari
From the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the calorimeter absorbs only a negligible quantity of heat, that the total volume of the solution is 100.0 ml, and that the specific heat and density of the solution after mixing are the same as that of pure water.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Do you know the correct answer?
From the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the cal...
Questions in other subjects:


English, 12.10.2019 10:10


Mathematics, 12.10.2019 10:10







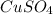 is 1.0 M and its volume is 50.0 ml or
is 1.0 M and its volume is 50.0 ml or 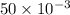 L (as 1 L = 1000 mL).
L (as 1 L = 1000 mL).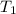 =
= 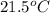 and
and 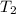 =
= 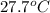
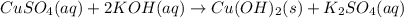
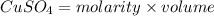
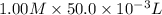
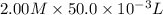
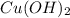 formed = moles
formed = moles 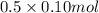
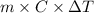
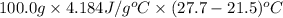
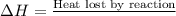
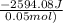
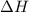 for the given reaction that occurs on mixing is -51.9 kJ/mol.
for the given reaction that occurs on mixing is -51.9 kJ/mol. 




