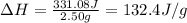Chemistry, 29.10.2019 07:31, acavalieri72
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temperature of the water decreased by 0.93 oc. the heat capacity of the calorimeter is 42.2 j/oc. the density of the water (and the solution) is 1.00 g/ml. the specific heat capacity of the solution is 4.184 j/goc. calculate the enthalpy change for dissolving this salt on a energy per mass basis (units of j/g).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, fgcherubin
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3
Do you know the correct answer?
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temp...
Questions in other subjects:

History, 06.03.2020 11:26










![q=[q_1+q_2]](/tpl/images/0350/9166/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0350/9166/1d50b.png)
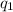 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter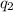 = heat absorbed by the water
= heat absorbed by the water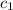 = specific heat of calorimeter =
= specific heat of calorimeter = 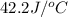
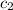 = specific heat of water =
= specific heat of water = 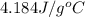
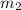 = mass of water =
= mass of water = 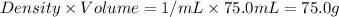
 = change in temperature =
= change in temperature = 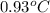
![q=[(42.2J/^oC\times 0.93^oC)+(75.0g\times 4.184J/g^oC\times 0.93^oC)]](/tpl/images/0350/9166/57473.png)
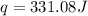
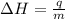
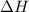 = enthalpy change = ?
= enthalpy change = ?