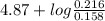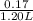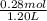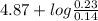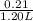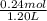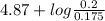Chemistry, 29.10.2019 05:31, stuckonquestions
Abuffer contains 0.19 mol of propionic acid (c2h5cooh) and 0.26 mol of sodium propionate (c2h5coona) in 1.20 l. you may want to reference (pages 721 - 729) section 17.2 while completing this problem. part a what is the ph of this buffer? express the ph to two decimal places. php h = nothing request answer part b what is the ph of the buffer after the addition of 0.02 mol of naoh? express the ph to two decimal places. php h = nothing request answer part c what is the ph of the buffer after the addition of 0.02 mol of hi? express the ph to two decimal places.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Do you know the correct answer?
Abuffer contains 0.19 mol of propionic acid (c2h5cooh) and 0.26 mol of sodium propionate (c2h5coona)...
Questions in other subjects:


Business, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00


Physics, 20.02.2021 01:00

History, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00



Mathematics, 20.02.2021 01:00


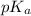 of propionic acid = 4.87
of propionic acid = 4.87
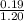
![pK_{a} + log(\frac{[salt]}{[acid]})](/tpl/images/0350/6880/fe481.png)