
Chemistry, 29.10.2019 04:31, AdanNava699
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
excess ni2+) to convert the cyanide totetracyanonickelate(ii):
4cn- + ni2+ (cn)42-
the excess ni2+ was then titrated with 10.15 ml of0.01307 m acid
(edta). one mole of this reagent reacts with 1 mol ofni2+:
ni2+ + edta4- (edta)2-
ni(cn)42- does not react with edta. if 39.35ml of the 0.01307 m edta was required to react
with 30.10 ml of the original ni2+ solution, calculatethe molarity of cn- in the 12.73 ml cyanide
sample.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2



Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
Questions in other subjects:





Social Studies, 12.09.2019 20:10

History, 12.09.2019 20:10



Biology, 12.09.2019 20:10

English, 12.09.2019 20:10


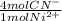 = 1,178x10⁻³ moles of CN⁻
= 1,178x10⁻³ moles of CN⁻




