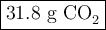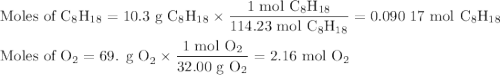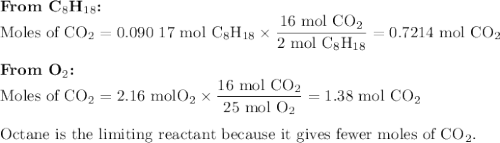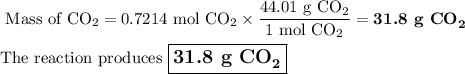Chemistry, 29.10.2019 04:31, iamabouttofail
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 10.3 g of octane is mixed with 69. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3


Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
Do you know the correct answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions in other subjects:

Mathematics, 19.11.2020 18:20

Chemistry, 19.11.2020 18:20

Physics, 19.11.2020 18:20

Mathematics, 19.11.2020 18:20

History, 19.11.2020 18:20

English, 19.11.2020 18:20


English, 19.11.2020 18:20