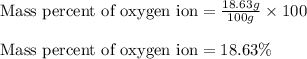In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing chloride ion and waters of hydration, was analyzed, and the following results were obtained. a 0.256-g sample of the compound was dissolved in water, and excess silver nitrate was added. the silver chloride was filtered, dried, and weighed, and it had a mass of 0.308 g. a second sample of 0.416 g of the compound was dissolved in water, and an excess of sodium hydroxide was added. the hydroxide salt was filtered and heated in a flame, forming cobalt(iii) oxide. the mass of the cobalt(iii) oxide formed was 0.145 g. what is the percent composition, by mass, of the compound?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
Do you know the correct answer?
In most of its ionic compounds, cobalt is either co(ii) or co(iii). one such compound, containing ch...
Questions in other subjects:

Biology, 22.11.2019 18:31




English, 22.11.2019 18:31

Social Studies, 22.11.2019 18:31




Mathematics, 22.11.2019 18:31


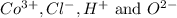 ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively
ions in the compound are 49.28 %, 29.79 %, 2.33 % and 18.63 % respectively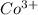 ions = 0.416 grams
ions = 0.416 grams ......(1)
......(1)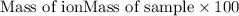 .......(2)
.......(2)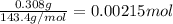

 of chloride ions
of chloride ions
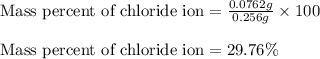
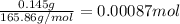

 of cobalt ions
of cobalt ions