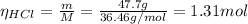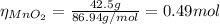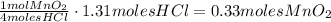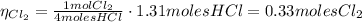Chemistry, 26.10.2019 04:43, jamaiciaw6
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(iv) oxide:
4hcl(aq) + mno2(s) > mncl2(aq) + 2h2o(l) + cl2(g)
you add 42.5 g of mno2 to a solution containing 47.7 g of hcl.
(a) what is the limiting reactant? mno2 or hcl?
(b)what is the theortical yield of co2?
(c) if the yield of the reaction is 79.9%, what is the actual yield of chlorine?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1


Do you know the correct answer?
Chlorine gas can be prepared in the laboratory by the reaction of hydrochloric acid with manganese(i...
Questions in other subjects:

Mathematics, 18.12.2020 03:40






Mathematics, 18.12.2020 03:40


Physics, 18.12.2020 03:40

Health, 18.12.2020 03:40