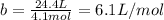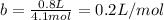Chemistry, 26.10.2019 03:43, barnhill6534
It turns out that the van dar waals constant b is equal to four times the total volume actually occupied by the molecules of a mole of gas. using this figure, calculate the fraction of the volume in a container actually occupied by ar atoms:
a) at stp
b) at 100 atm pressure and 0 degrees celsius

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Do you know the correct answer?
It turns out that the van dar waals constant b is equal to four times the total volume actually occu...
Questions in other subjects:




Mathematics, 04.09.2020 16:01

Mathematics, 04.09.2020 16:01

Biology, 04.09.2020 16:01





 (1)
(1)