
Chemistry, 26.10.2019 03:43, Deavionaaaaa
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relaxes back to the ground state by emitting two photons, the first, a red photon at 700 nm, and the second, an infrared photon at 1750 nm. what is the wavelength of the absorbed photon? 500 nm 1225 nm 700 nm 1950 nm 1750 nm

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 23:00, catdog5225
What is formed when amino acids form long chains or polymerize
Answers: 1
Do you know the correct answer?
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relax...
Questions in other subjects:

Mathematics, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

History, 18.10.2020 09:01

World Languages, 18.10.2020 09:01

Mathematics, 18.10.2020 09:01

Computers and Technology, 18.10.2020 09:01


Mathematics, 18.10.2020 09:01

Spanish, 18.10.2020 09:01

History, 18.10.2020 09:01


 ) = 700 nm
) = 700 nm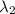 ) = 1750 nm
) = 1750 nm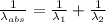
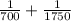
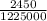
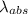 =
= 




