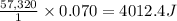Chemistry, 26.10.2019 00:43, tmanpierce
Astudent enters the lab and conducts part a of the experiment. the student uses 32.00 ml of 2.202 m hcl, and adds naoh in excess as instructed. if the δh of the neutralization reaction is known to be -57,320 j/mol h2o, what is the total theoretical heat released (in joules)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, faithrawlins14
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Do you know the correct answer?
Astudent enters the lab and conducts part a of the experiment. the student uses 32.00 ml of 2.202 m...
Questions in other subjects:

Mathematics, 12.12.2020 15:50


Biology, 12.12.2020 15:50

History, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50




Mathematics, 12.12.2020 15:50



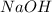 is in excess ,
is in excess , 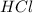 is the limiting reagent as will decide the amount of product formed and energy released.
is the limiting reagent as will decide the amount of product formed and energy released.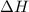 = -57,320 J/mol
= -57,320 J/mol