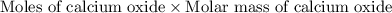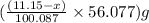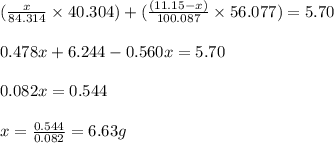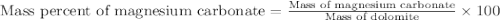Chemistry, 26.10.2019 00:43, mckadams02
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides mgo and cao upon heating. when 11.15 g of dolomite is heated, 5.70 g of mgo and cao are produced. what is percent by mass of mgco3 in the original sample of dolomite?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kylieweeks052704
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 21.06.2019 23:30, tylerineedhelp
Ihat will happen if i added baking soda to vinegar
Answers: 2


Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
Do you know the correct answer?
Dolomite is a mixed carbonate of calcium and magnesium that decomposes to co2 and the metal oxides m...
Questions in other subjects:




Mathematics, 18.03.2020 22:35







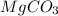 in dolomite is 59.5 %
in dolomite is 59.5 %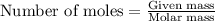
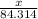 moles
moles 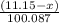 moles
moles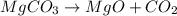
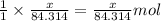 of magnesium oxide
of magnesium oxide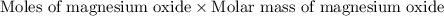
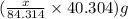

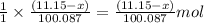 of calcium oxide
of calcium oxide