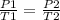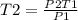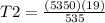Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
Do you know the correct answer?
Using the same sample of gas (p1 = 535 torr , t1 = 19 ∘c ), we wish to change the pressure to 5350 t...
Questions in other subjects:

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

English, 20.09.2020 22:01

English, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

English, 20.09.2020 22:01