Chemistry, 25.10.2019 19:43, niceguy1997
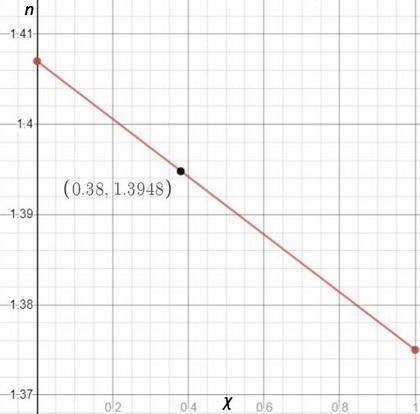
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components, hexane and nonane you place 15 ml of the mixture in a round bottom flask, and then collect the distillate sequentially as four three ml samples labeled s1, s2, s3, and s4. pure hexane has a refractive index of 1.375 and pure nonane has a refractive index of 1.407. you measure a refractive index of 1.3948 for one of the four samples. assuming the refractive index varies linearly with mole fraction, estimate the mole fraction of hexane in this sample.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jetblackcap
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
You are performing a simple distillation of roughly 50: 50 liquid solution containing two components...
Questions in other subjects:



Mathematics, 24.02.2021 19:20


Physics, 24.02.2021 19:20

Chemistry, 24.02.2021 19:20


Mathematics, 24.02.2021 19:20

Mathematics, 24.02.2021 19:20