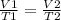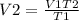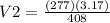Calculate the missing variables below using charles's law
v1 = 3.17 l, t1 = 408 k
...

Chemistry, 25.10.2019 01:43, giordanolucia18
Calculate the missing variables below using charles's law
v1 = 3.17 l, t1 = 408 k
v2 = ? , t2 = 277 k
a. 2.15 l
b. 4.67 l
c. 0.214 l
d. no right answer.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Engineering, 27.04.2022 14:00





Mathematics, 27.04.2022 14:50


Mathematics, 27.04.2022 15:00

Mathematics, 27.04.2022 15:00