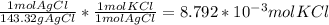Chemistry, 25.10.2019 00:43, cookiebrain72
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample of impure potassium chloride (0.900 g) was dissolved in water, and treated with excess silver nitrate (agno3), 1.26 g of silver chloride (agcl) was precipitated. calculate the percentage kcl in the original sample.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
Do you know the correct answer?
Consider the following balanced chemical equation, kcl(aq) agno3(aq) → agcl(s) kno3(aq)when a sample...
Questions in other subjects:

Mathematics, 06.10.2021 14:40







Mathematics, 06.10.2021 14:40


Social Studies, 06.10.2021 14:40