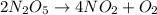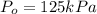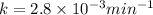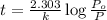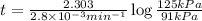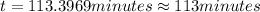The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, the rate constant is 2.8 × 10-3min-1. if a rigid vessel initially contains only n2o5 at a pressure of 125 kpa, how long will it take for the total pressure to reach 176 kpa? options (pick 1)a)113 minb)129 minc)42 mind)182 mine)62 minf)83 min

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
Do you know the correct answer?
The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, t...
Questions in other subjects:


Mathematics, 20.08.2020 01:01

English, 20.08.2020 01:01

English, 20.08.2020 01:01



Mathematics, 20.08.2020 01:01