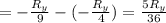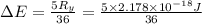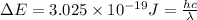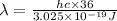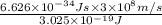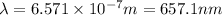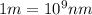The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
Questions in other subjects:



Mathematics, 22.09.2019 14:50


English, 22.09.2019 14:50


Social Studies, 22.09.2019 14:50


Social Studies, 22.09.2019 14:50

English, 22.09.2019 14:50


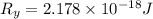
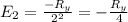
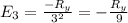
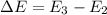 :
: