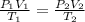Chemistry, 24.10.2019 17:43, dominaricann2451
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the reaction of nitrogen oxide with oxygen. for each (1.0) mole of no: i. how many moles of o_2 are consumed and of no_2 produced? ii. how many liters of each (state conditions)? iii. how many grams of each? d. calculate the mass of 4.55 l of hci gas at stp. e. find the molecular mass of a gas having a density of a gas having a density of 0.00481 g/ml at stp. f. write a balanced equation for the reaction you caused to occur in this assignment. solve the following problems, assume that you used a sample with a mass of 2.550 grams. i. if the sample was pure kcio_3 and it was completely decomposed into kci and o_2, fill in the following blanks: there will be moles of o_2 obtained, having a mass of grams and occupying ml at stp or ml at 29 degree c and 732 torr. ii. assume now that the sample was an unknown mixture of kcio_3 with kci and that complete decomposition yielded 182 ml of oxygen gas measured at 29 degree c and 732 torr. calculate the percent by mass of kcio_3 in the mixture. g. nitrogen gas was collected over water at 26 degree c and 755 torr. if the volume collected was 35.9l. what volume would it occupy, dry, at stp?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, josephaciaful
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1


Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
Do you know the correct answer?
The formula of nitrogen oxide is no, of nitrogen dioxide is no_2. write a balanced equation for the...
Questions in other subjects:



Social Studies, 13.10.2019 00:00



Mathematics, 13.10.2019 00:00


English, 13.10.2019 00:00

Mathematics, 13.10.2019 00:00


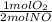 = 0,5 mol of O₂
= 0,5 mol of O₂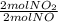 = 1 mol of NO₂
= 1 mol of NO₂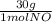 = 30g
= 30g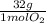 = 16g
= 16g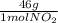 = 46g
= 46g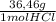 = 7,41 g of HCl
= 7,41 g of HCl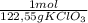 = 0,0208 moles of KClO₃
= 0,0208 moles of KClO₃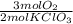 = 0,0312 moles of O₂
= 0,0312 moles of O₂ = 0,999g of O₂
= 0,999g of O₂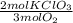 = 0,0106 mol KClO₃. In grams:
= 0,0106 mol KClO₃. In grams: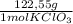 = 1,300 g of KClO₃.
= 1,300 g of KClO₃.