
Chemistry, 24.10.2019 03:00, morganlynn18
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (provides a -, the conjugate base of ha). pk a for ha is 6.23. how many moles of a - will be present in the mixture after addition of 0.20 ml 0.010 m naoh? (suggestion: start by calculating the moles of everything in the mixture.) enter the numerical value, without units, to at least 2 significant figures. you may use e-format scientific notation, e. g., 1.23x10 -4 would be entered as 1.23e-4. (note that the acid and base do not add up to 20.00 ml -- water must be added. that does not affect how you answer the question.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2
Do you know the correct answer?
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (prov...
Questions in other subjects:



Social Studies, 11.06.2020 02:57


Mathematics, 11.06.2020 02:57

History, 11.06.2020 02:57



Mathematics, 11.06.2020 02:57


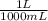 = 5x10⁻³ L of HA
= 5x10⁻³ L of HA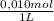 = 5x10⁻⁵ mol of HA
= 5x10⁻⁵ mol of HA




