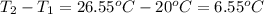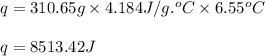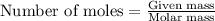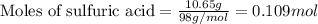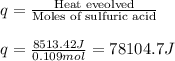When pure sulfuric acid is dissolved in water, heat is evolved. in a laboratory experiment to measure the molar heat of solution of sulfuric acid, the following procedure was followed. to a calorimeter containing 3.00 × 102 g of water at 20.00 °c, 10.65 g of h2so4, also at 20.00 °c was added. the temperature change, which was monitored by a digital thermometer with negligible heat capacity, ceased when it reached a temperature of 26.55 °c. if the specific heat of the mixture is 4.184 j g‑1 °c‑1, and the small heat capacity of the calorimeter is ignored, what is the heat evolved, per mole of sulfuric acid? show an overview of your work.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
Do you know the correct answer?
When pure sulfuric acid is dissolved in water, heat is evolved. in a laboratory experiment to measur...
Questions in other subjects:

English, 12.02.2021 23:20



History, 12.02.2021 23:20

Mathematics, 12.02.2021 23:20

Mathematics, 12.02.2021 23:20

English, 12.02.2021 23:20

Mathematics, 12.02.2021 23:20

English, 12.02.2021 23:20

Medicine, 12.02.2021 23:20


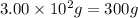
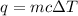
 = change in temperature =
= change in temperature =