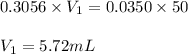Chemistry, 23.10.2019 22:30, Bryanguzman2004
You wish to prepare 50.00 ml of 0.0350 m nh3 from concentrated ammonia (15.28 m) by serial dilution using a 50 ml volumetric flasks, a 1.00 ml volumetric pipet, and a 10.00 ml graduated pipet. for the first dilution in the series, 1 ml of concentrated ammonia is added to a 50.00 volumetric flask and then filled to the line. the solution is mixed thoroughly, poured into a beaker, and then used to make the second solution in the series. what volume of the first solution should be used to make the second solution, 50.00 ml of 0.0350 m nh3 solution, using the glassware provided?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, qwerty8364
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1


Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
Do you know the correct answer?
You wish to prepare 50.00 ml of 0.0350 m nh3 from concentrated ammonia (15.28 m) by serial dilution...
Questions in other subjects:


Chemistry, 23.09.2019 12:30








History, 23.09.2019 12:30


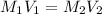 .......(1)
.......(1)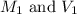 are the molarity and volume of the concentrated ammonia solution
are the molarity and volume of the concentrated ammonia solution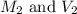 are the molarity and volume of diluted ammonia solution
are the molarity and volume of diluted ammonia solution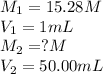
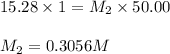
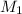 = Molarity of ammonia solution from first dilution = 0.3056 M
= Molarity of ammonia solution from first dilution = 0.3056 M = Volume of ammonia solution from first dilution = ?
= Volume of ammonia solution from first dilution = ?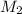 = Molarity of ammonia to make second solution = 0.0350 M
= Molarity of ammonia to make second solution = 0.0350 M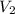 = Volume of ammonia to make second solution = 50.00 mL
= Volume of ammonia to make second solution = 50.00 mL