
Chemistry, 23.10.2019 20:00, hetdashadia123
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reactions: 3c2h4 + 2h2so4 → c2h5hso4 + (c2h5)2so4 c2h5hso4 + (c2h5)2so4 + 3h2o → 3c2h5oh + 2h2so4 what volume of ethylene at stp is required to produce 1.000 metric ton (1000 kg) of ethanol if the overall yield of ethanol is 90.1%

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
Do you know the correct answer?
Ethanol, c2h5oh, is produced industrially from ethylene, c2h4, by the following sequence of reaction...
Questions in other subjects:

Mathematics, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20





Mathematics, 29.01.2021 21:20




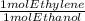 * 100/90.1 = 2.4128* 10⁴ mol ethylene
* 100/90.1 = 2.4128* 10⁴ mol ethylene




