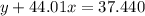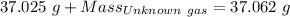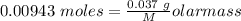Chemistry, 23.10.2019 20:30, camirialchambers17
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the same container filled with carbon dioxide at stp has a mass of 37.440 g. when filled with an unknown gas at stp, the container mass is 37.062 g. calculate the molecular weight of the unknown gas, and then state its probable identity.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jamccoy3335
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
Do you know the correct answer?
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the...
Questions in other subjects:

Mathematics, 22.03.2021 22:50

Mathematics, 22.03.2021 22:50

English, 22.03.2021 22:50

Mathematics, 22.03.2021 22:50

Mathematics, 22.03.2021 22:50


English, 22.03.2021 22:50

Spanish, 22.03.2021 22:50

History, 22.03.2021 22:50


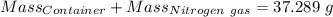
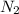 = 28.014 g/mol
= 28.014 g/mol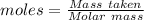
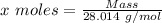
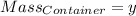
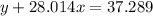
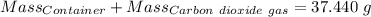
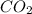 = 44.01 g/mol
= 44.01 g/mol