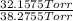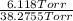Chemistry, 23.10.2019 19:20, SavgeKid7578
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour pressure of pure ccl4 is 33.85 torr at 298k. the henry’s law constant when the concentration of br2 is expressed as a mole fraction is 122.36 torr. calculate the vapour pressure of each component, the total pressure, and the composition of the vapour phase when the mole fraction of br2 is 0.050, on the assumption that the conditions of the ideal-dilute solution are satisfied at this condition

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1



Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
Adilute solution of bromine in carbon tetrachloride behaves as an ideal-dilute solution. the vapour...
Questions in other subjects:


Mathematics, 05.04.2021 07:40

English, 05.04.2021 07:40



Chemistry, 05.04.2021 07:40

Mathematics, 05.04.2021 07:40

Mathematics, 05.04.2021 07:40

Biology, 05.04.2021 07:40

Biology, 05.04.2021 07:40


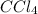 = 33.85 Torr
= 33.85 Torr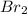 = 122.36 torr
= 122.36 torr