
The sulfur content of an ore is determined gravimetrically by reacting the ore with concentrated nitric acid and potassium chlorate, converting all sulfur to sulfate. the excess nitrate and chlorate is removed by reaction with concentrated hydrochloric acid and the sulfate is precipitated using barium cation.
ba2+ (aq) + so42- (aq) = baso4 (s)
analysis of 12.3430 grams of a sulfur containing ore yielded 12.5221 grams of baso4. what is the percent by mass sulfur in the ore? (baso4 = 233.43 g/mol). show all calculation.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
Do you know the correct answer?
The sulfur content of an ore is determined gravimetrically by reacting the ore with concentrated nit...
Questions in other subjects:

Biology, 23.11.2020 06:00

English, 23.11.2020 06:00



Physics, 23.11.2020 06:00


Mathematics, 23.11.2020 06:00

Mathematics, 23.11.2020 06:00


Mathematics, 23.11.2020 06:00


 = 12.5221 g
= 12.5221 g
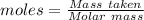


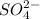
 =
= 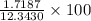 = 13.92 %
= 13.92 %



