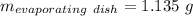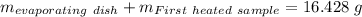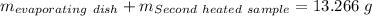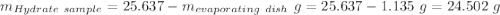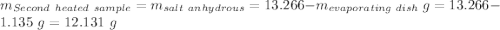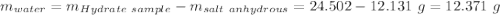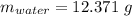Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.428 g. after a second heating cycle, the mass of the sample and the dish is 13.266 g. the student stops the heat/cool/weigh cycle after the second time. if the original mass of the evaporating dish and the hydrate was 25.637 g, and the mass of the evaporating dish alone was 1.135 g, what is the mass of water removed from the sample by heating? provide your response to three digits after the decimal.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
Do you know the correct answer?
Astudent heats a sample of hydrate once, and the mass of the sample and the evaporating dish is 16.4...
Questions in other subjects:


History, 20.08.2019 21:50



Chemistry, 20.08.2019 21:50


English, 20.08.2019 21:50